Summary
The tagging of 2,5-dimethoxyphenylisopropylamine (DPIA) with bromine-77 (T½ = 56 h) was reinvestigated in order to achieve high specific activities which are necessary for in-vivo application of this centrally acting drug. A fast one-pot synthesis was developed using N-chlorotetrafluorosuccinimide (NCTFS) and practically carrier-free bromide to introduce radiobromide into the aromatic ring. Trifluoroacetic anhydride (TFA) was applied both as solvent and as a reversible blocking agent of the free amino group. TFA was also shown to suppress chlorinating side-reactions. Rapid separation of 4-77Br-2,5-dimethoxyphenylisopropylamine was achieved by reverse phase high pressure liquid chromatography. The total labelling and separation procedure takes about two hours, and 4-77Br-DOB is obtained with an overall radiochemical yield of 25% and a specific activity of >8 Ci/µmole.
Introduction
The amphetamine analogue 2-(4-bromo-2,5-dimethoxyphenyl)-isopropylamine (DOB) was found by Shulgin, Sargent and Naranjo to be a highly active psychotomimetic agent1. Oral and i.v. application of the radiobromine labelled drug to man demonstrated its active take up by different organs, especially by the brain2. It was therefore suggested as a potential brain scanning agent3.
77Br-DOB

Labelling of the drug with bromine-77 is motivated by two reasons. It has been observed that substitution of hydrogen in the 4-position of 2,5-dimetoxyphenylisopropylamine (DPIA) by a methoxy group (TMA-2) or a methyl group (STP) causes an enhancement of central activity by a factor of 2 and 10, respectively4. Substitution of the methyl group by the isosteric bromine intensifies the psychotomimetic activity by a factor of hundred which means that it is 400 times more active than mescaline1. The high activity is explained by the structural relation to 6-hydroxydopamine and the hindered metabolic attack at the 4-position. Secondly, maximum accumulation in the brain occurs 3 to 6 hours after application; thus among the potentially useful neutron deficient halogen isotopes the longer lived 77Br (T½ 56 h) is preferable. But also the shorter lived positron emitter 75Br (T½ 98 min) might still be useful for positron emission tomography5.
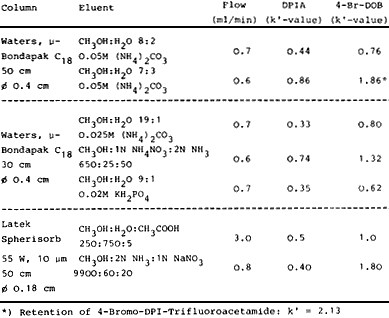
Table I.
Separation of 2,5-Dimethoxyphenylisopropylamine
(DPIA) from 4-Br-DOB on HPLC column.
Recently a labelling procedure was reported2, using 77Br-Br2 in relatively small specific activities of 57 mCi/mmol. This would necessitate the application of small activities and hence long time measurements when applied as a brain agent. The low psychotomimetic active dose of 0.2 to 2 mg per man and the desirable fast sequence of pictures for measurements of metabolic turnover, however, requires high specific activities. A re-investigation of the bromination of DPIA with the goal to obtain practically carrier-free products seemed therefore desirable. Theoretically carrier-free bromine labelled aromatic compounds can be obtained by a recently developed method using N-chlorotetrafluorosuccinimide (NCTFS) and carrier-free bromide-776. NCTFS has been used for labelling of monosubstituted benzenes starting with 77Br-bromide. High specific activities, relatively high radiochemical yields together with high para-selectivities (60-90%) had been observed. The application of the NCTFS-method to the bromination of DPIA was of special interest, since the amine acts as a scavenger for electrophilic species. Efficient labelling should be possible when using trifluoroacetic anhydride (TFA) as solvent which acted as reversible blocking agent of the amino group.
Experimental
Starting Materials
Carrier-free 77Br-bromide was produced via the 75As(α,2n)77Br nuclear reaction bombarding thick Cu3As-alloy targets at the Jülich Compact Cyclotron CV-28 (TCC) with 28 MeV α-particles. After isolation from the target material by a dry distillation technique7 the bromide was dissolved in triple distilled water. The 77Br-bromide-water solution was transferred to a reaction vessel and evaporated to dryness.
N-chlorotetrafluorosuccinimide (NCTFS) has been prepared via tetrafluorosuccinimide as described previously8. This compound was also obtained by the reaction of ammonia with tetrafluorosuccinic anhydride (Merck), isolation of the free succinic acid monoamide by HCl-treatment, and following cyclization by condensation with P2O5 under reduced pressure9. 2,5-Dimethoxyphenylisopropylamine (DPIA) was prepared by aldol condensation of 2,5-dimethoxybenzaldehyde and nitroethane using ammonium acetate in acetic acid10 and reduction of the resulting styrol to the amine (DPIA) by LiAlH4 in dry THF11. The free amine was purified by vacuum distillation [bp 120-122°C/1 mmHg] resulting in a colorless oil. Bromination of the amine gave rise to the 4-bromo-compound to be used as reference substrate for HPLC-separation (see below). Bromination was carried out with molecular bromine in acetic acid12 similar to the procedure reported. The identity and purity of the amino compounds were checked by 1H-NMR spectroscopy (Bruker, WP-80) and high pressure liquid chromatography.
High Pressure Liquid Chromatography
Separation and purification of the labelled compound has been performed by high pressure liquid chromatography. A reverse phase column packed with µ-Bondapak C-18 (Waters) or a polar column with silica gel (Latek) were used with different eluents. The columns were mounted to a Hewlett Packard liquid chromatograph 1010A with an UV detector from Waters Assoc. The identification of the labelled compound and the purity of the starting material (DPIA) have been tested using different columns and eluents. The conditions for the separations are listed in Table I.
For preparative separation of 4-77Br-DOB from the starting material (DPIA) the reverse phase column using a 0.03 molar (NH4)2CO3-solution of CH3OH-H2O (7:3) as eluent was best suited for this purpose. Earlier separations of amphetamine analogues13 using 2N ammonia - 1N ammonium nitrate - methanol eluents on silica gel columns lead only to poor resolutions.
4-77Br-2,5-Dimethoxyphenylisopropylamine
Eq. I

The optimized reaction conditions of the NCTFS-method have been described previously6. For the bromination of DPIA the corresponding trifluoroacetamide was employed, which was obtained by dissolving the amine in an excess of trifluoroacetic anhydride (TFA) while cooling (Eq. I).
TFA serves as blocking agent for the amine group and as solvent. It was observed that a chlorinating side reaction (possibly via NCTFS) leading to non-isotopic carrier does not occur in pure anhydride solution6.
Eq. II

0.5 ml of TFA containing 5 to 10 mg of the amine (DPIA) was given to a 2 ml reaction vessel with carrier-free, dry 77Br-bromide and 2 mg NCTFS, weighed in a dry-box. The bromination reaction was allowed to take place in darkness at room temperature and gave rise to the 4-77Br-acetamide compound (4-77Br-AA) (Eq. II).
Eq. III

After one hour the TFA was evaporated. The residue was dissolved in aqueous NaOH solution at pH 10 and warmed for 1 hour (or milder at pH 8 at room temperature for 12 hours) in order to hydrolyze the acetamide (Eq. III). The liberated 77Br-amine was extracted with CCl4 (3x0.5 ml), isolated by evaporation of the solvent and redissolved in methanol. The resulting 77Br-4-bromo-amphetamine was separated by HPLC (see above). The whole labelling procedure took less than 2 hours and exclusively lead to the para product. The overall radiochemical yield of 4-77Br-DOB was 25% after chromatographic separation.
During HPLC analysis no UV-signal of 4-Br-DOB could be detected. Even if a bromide contamination from reagents in the order of 0.1 mg would be present, a specific activity of 8 Ci/µmole is obtained. This specific activity allows in vivo application of higher total activities without toxicity problems.
The positive results suggest that the NCTFS-method can be applied to other complicated biomolecules, especially amino acids and oligopeptides which are of interest since they can be protected reversibly by TFA under mildest conditions14.