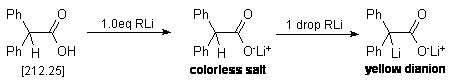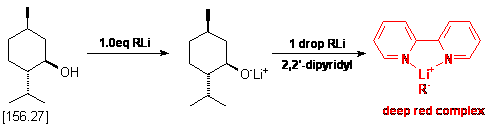An accurate concentration of alkyllithium reagents is essential for performing
air and moisture sensitive chemistry. Thus, it is essential that periodic assays
of the alkyllithium reagents be performed to ensure quality results in reactions
which require the use of such chemicals. The following procedures are for the
titrations of these organometallics. These titrations are usually performed
three times and an average of the concentrations is taken to be certain that
an accurate titer is obtained.
Procedure 1
Titrations of organometallics using diphenylacetic acid
An oven-dried 25 mL three-necked round bottom flask equipped with a nitrogen
inlet, a stirring bar and two rubber septa is cooled under a stream of nitrogen.
The flask is then charged with ca. 300 mg (but accurately weighed
out!*) of diphenylacetic acid and 10 mL of dry THF. To the resulting
solution is added the organometallic reagent (alkyl lithiums) via a 1.0 mL syringe
(graduated in 0.01 mL increments) in a dropwise fashion. During addition, it
will be observed that periodic quantities of a yellow colored substance will
appear in solution. This is the enolate dianion of diphenyl acetic acid, and
it will be noted that in the early stages of this titration, this yellow color
will disperse rapidly. As one nears the endpoint, the yellow coloration will
require longer periods of time to disperse. At this point, it is necessary to
slow the rate of addition of the alkyl lithium reagent as the endpoint is approaching.
Eventually, the addition of a single drop of alkyl lithium will cause a persistent
yellow coloration of the solution. This is the endpoint of the titration. Take
note of the final volume of the syringe. The difference between the original
volume of organometallic solution and this final volume of solution represents
the volume of the titrant. Repeat this titration two additional times using
a fresh flask, solvent and diphenyl acetic acid. The average of these three
runs constitutes the molarity of the alkyl lithium solution.
Shown below is the chemical reaction which is occurring during the titration,
as well as a sample calculation for determining the molarity of the alkyl lithium
solution. In this example, 300mg of diphenylacetic acid was used, which required
0.84mL of the RLi solution to reach endpoint.
0.300g acid = 1.40mmol acid, which reacts with 1.40mmol
RLi;
this amount of RLi was present in 0.84mL of the analyte.
Since molarity equals moles/L, it also equals mmol/mL. Thus:
1.40 mmol RLi / 0.84mL solution = 1.67M RLi soln.
* In other words, you should use about 300mg of diphenylacetic acid,
but it doesn't have to be
exactly 300mg. However, you must know, precisely, the amount
that you do use, since it is
from this value that the alkyl lithium molarity is being calculated!
Procedure 2
Titration of alkyl lithium solutions using a charge transfer complex indicator
This procedure is slightly superior to the above method due to the fact that
the endpoint is colored bright red, which makes distinguishing the endpoint
much easier. This procedure is almost identical to the one mentioned above.
An oven dried 25 mL three neck flask equipped with a nitrogen inlet adapter,
a stirring bar and two rubber septa was cooled under a stream of nitrogen. Upon
cooling the flask was charged with ca. 200 mg of menthol (but accurately
weighed out!), ca. 5 mg of 2,2'-dipyridyl and 10 mL of dry THF.
To the resulting solution is added the organometallic reagent (alkyl lithiums)
via a 1.0 mL syringe (graduated in 0.01 mL increments) in a dropwise fashion.
During addition, it will be observed that periodic quantities of a red colored
substance will appear in solution. This is the charge transfer complex between
the alkyl lithium and 2,2'-dipyridyl, and it will be noted that in the early
stages of this titration this red color will disperse rapidly. As one nears
the endpoint, the red coloration will require longer periods of time to disperse.
At this point, it is necessary to slow the rate of addition of the alkyl lithium
reagent as the endpoint is approaching. Eventually, the addition of a single
drop of alkyl lithium will cause a persistent red coloration of the solution.
This is the endpoint of the titration. Take note of the final volume of the
syringe. The difference between the original volume of organometallic solution
and this final volume of solution represents the volume of the titrant. Repeat
this titration two additional times using a fresh flask, solvent, 2,2'-pyridyl,
and menthol. The average of these three runs constitutes the molarity of the
alkyl lithium solution.
Shown below is the chemical reaction which is occurring during the titration,
as well as a sample calculation for determining the molarity of the alkyl lithium
solution. Note that the calculation is virtually identical to that done in Procedure
1. In this example, 200mg of menthol was used, which required 0.73mL of the
RLi solution to reach endpoint.
0.200g menthol = 1.30mmol menthol, which reacts with 1.30mmol
RLi;
this amount of RLi was present in 0.73mL of the analyte.
Since molarity equals moles/L, it also equals mmol/mL. Thus:
1.30 mmol RLi / 0.73mL solution = 1.78M RLi soln.
Procedure 3
Titration of potassium tert-butoxide solutions using an acid/base indicator
The following procedure may be used to titrate either LDA solutions or potassium
tert-butoxide solutions in THF. The procedure is very similar to those
listed above with one notable exception: the indicator used here is fluorene,
which is sufficiently acidic that it can be deprotonated by weak bases such
as metal alkoxides.
An oven-dried 25 mL three-necked flask equipped with a nitrogen inlet adapter,
a stirring bar and two rubber septa was cooled under a stream of nitrogen. Upon
cooling the flask was charged with ca. 200 mg of menthol (but accurately
weighed out!), ca. 5 mg of fluorene and 10 mL of dry THF. To
the resulting solution is added the potassium tert-butoxide solution
via a 1.0 mL syringe (graduated in 0.01 mL increments) in a dropwise fashion.
During addition, it will be observed that periodic quantities of a yellow colored
substance will appear in solution. This is the deprotonated form of fluorene,
and it will be noted that in the early stages of this titration this yellow
color will disperse rapidly. As one nears the endpoint, the yellow coloration
will require longer periods of time to disperse. At this point, it is necessary
to slow the rate of addition of the alkoxide solution as the endpoint is approaching.
Eventually, the addition of a single drop of alkoxide will cause a persistent
yellow coloration of the solution. This is the endpoint of the titration. Take
note of the final volume of the syringe. The difference between the original
volume of alkoxide solution and this final volume of solution represents the
volume of the titrant. Repeat this titration two additional times using a fresh
flask, solvent, fluorene, and menthol. The average of these three runs constitutes
the molarity of the potassium tert-butoxide solution. The calculation
is identical to that shown for Procedure 2.