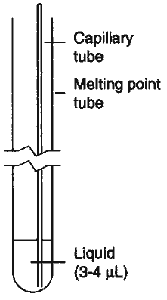
At the microscale level, the boiling point of a liquid may be determined using only 3-4 µL of liquid1. The liquid is added to a melting-point tube using a 10-µL syringe and centrifuged to the bottom, if necessary. After a 5-mm-long glass bell is inserted, open end down, the tube is placed in a conventional melting-point apparatus (e.g., Mel-Temp). The temperature is raised rapidly to 15-20°C below the expected boiling point and then increased at a rate of about 2°C/min. When the boiling point is reached, the fine stream of bubbles. stops as the vapor pressure of the liquid reaches atmospheric pressure1. A simple modification to this procedure alleviates many of the frustrations associated with the use of the glass bell (Fig. 1). A very fine capillary tube, prepared from a glass Pasteur pipet and sealed at one end, is much easier to insert into the melting-point tube and does not lift out of the liquid as the bubbles form. The glass fiber still attached to the glass pipet may be used instead of a more expensive syringe to transfer a slug of liquid into the bottom of the melting-point tube. The assembled system is then placed in the melting-point apparatus and the boiling point of the liquid determined as usual1. Since the bubble generator is so thin, a stream of very small bubbles is produced and no problem is experienced with the liquid creeping up the tube because of capillary action.