Abstract
In this review, attention is paid to chromatographic and mass spectral properties of already identified impurities found to be present in frequently abused drug preparations of illegal origin of amphetamine and methamphetamine. The most commonly employed methods of synthesis of drugs of this type are briefly described. Special emphasis is given to the Leuckart route, found to be the preferred method, in the illicit production of amphetamine. Furthermore, some isolation and preconcentration methods for the contaminants are discussed. The importance of identifying impurities present in amphetamine or methamphetamine cannot be overestimated. These impurities originate mostly from the improper purification in the end stage of the different syntheses used in the clandestine manufacture of the substances; it is possible to differentiate between the several kinds of illegal drug preparations, synthesized by various methods, by means of so-called "route specific" impurities. Finally, a survey is given of the impurities already known to be present in amphetamine and methylamphetamine, together with their mass spectral and some chromatographic properties.
Introduction
A recurrent theme in forensic drug analysis concerns the possibilities of locating sources of supply and manufacture of illicit drugs by means of diagnostic chemical or physical properties17,38. Powder formulated drugs and their potential accompanying substances can be examined only on the basis of their chemical properties; whereas with tablets or capsules their visual appearance and the nature of bulking, binding, lubricating, diluting and coloring agents can play an important role.
In contrast to genuine drugs, illegal drug preparations are often contaminated. Impurities in these preparations largely depend on inadequate purification procedures, and they can originate from a variety of causes such as imperfect chemical handling, starting materials, side, and subsequent reactions, intermediate products, diluents, laboratory dirt, and from handling and packing the drugs. It stands to reason that chromatographic patterns obtained from illegally produced drugs might contain valuable information about the drug and its method of synthesis, even if the identity of only a limited number of the chromatographic peaks is known. In general, only patterns are compared and, if possible, recognized in these so-called "chemical signature" analyses38. In recent years some basic work has been done23,35 regarding the nature of contaminations encountered in the different syntheses of amphetamine or methamphetamine. Mostly it is related to the Leuckart reaction as the most popular method for the production of amphetamine in both the western European countries and the U.S.8,35.
In this review, the syntheses commonly used in the clandestine preparation of amphetamine and methamphetamine are discussed, followed by some remarks on the isolation of impurities from reaction mixtures and final preparations. Lastly some chromatographic and mass spectrometric data of the various impurities are given. The impurities present in amphetamine and methamphetamine are accentuated here, since the contaminants present can give important indications about the type of synthesis used. A contribution has been made in improving the information content of the "signature" analysis of amphetamines. Today even the presence of "reaction specific" impurities can be established in the chromatographic profiles.
Due to the scarcity of data, little attention can be given to inorganic impurities found in clandestine amphetamine or methamphetamine originating from hydrogenating catalysts.
Synthesis of Amphetamine
Over the years the Leuckart reaction has remained the most popular method for synthesizing illicit amphetamine in the U.S.8, the U.K.32, and The Netherlands. The reductive amination of benzyl methyl ketone is important8,16, while in Sweden and the U.S., the nitropropene route is incidentally used, like the phenyloxime route in the U.S. The following are some methods for the production of amphetamine, in which the chemical handling is not difficult and the necessary materials are easy to purchase. Many other methods are known, but are of lesser importance in this context. For further information refer to reference32.
A. The Leuckart Rection
This reaction can be formulated by the following scheme:
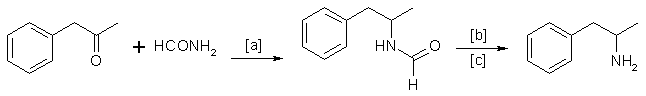
in which [a]: 180-190°C; [b]: H2SO4/HCl (dilute); [c]: 90-125 °C.
Reaction conditions can vary29. A trend15 in recent years has been to replace formamide by ammonium formate48 or a mixture of ammonia and formic acid6.
B. The Reductive Amination of Benzyl Methyl Ketone

Benzyl methyl ketone can react with ammonia in the following way:13
in which [a]: Raney Nickel, Pt, H2, Al powder in the presence of HgCl2, Nickel plated Zinc; [b]: 20-170°C, 1-130 atm, ethanol, methanol.
Reaction conditions can differ widely1,5,11,36. (Only low pressure and low temperature aminations have been encountered so far in the Netherlands.)
C. The Oxime Route
Benzyl methyl ketone reacts with hydroxylamine to give the oxime, which can be hydrogenated to give the amphetamine:14
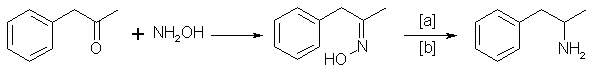
in which [a]: Na (amalgamated), Na (absolute ethanol), LiAlH4, or H2 and Raney Nickel, nickel, iron, nickel plated zinc; [b]: 20-170°C, 1-130 atm;
Electrolytical reduction has also been reported. Great differences have been described for the reaction conditions18,27,31.
D. The Phenylnitropropene Route
Condensation of benzaldehyde with nitroethane yields 1-phenyl-2-nitropropene2,9. Hydrogenation of the double bond and subsequent reduction of the nitro group gives the amphetamine:
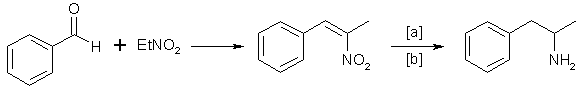
in which [a]: LiAlH4, H2 and Raney Nickel or Pd/C; [b]: 20-100°C, 1-80 atm, CH3OH, C2H5OH, H2O/HCOOH, C2H5OH.
Reaction conditions vary widely4,10.
Synthesis of Methamphetamine
In contrast to the western European countries, in the U.S. the illicit production of methamphetamine has preference over clandestine amphetamine production. (In The Netherlands, methamphetamine has rarely been produced up to the current time. Only once in the past 10 years has a high pressure reductive amination of benzyl methyl ketone with methylamine been found46, operating under near-professional standards.)
A. The Reductive Amination of Benzyl Methyl Ketone
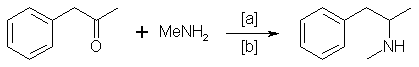
Illicit methamphetamine is primarly produced in the U.S.8 by reductive amination, according to the following scheme:
in which [a]: HgCl2/Al, NaBH4 in slightly acid medium, H2/Pd, Na/ethanol, H2, Raney Nickel; [b]: 25-160 °C, 1200 atm, methanol, ethanol, ethyl ether. Reaction conditions vary widely.
B. The Leuckart Reaction
In methamphetamine preparation, the following reaction is of minor importance, compared with reductive amination. Schematically:
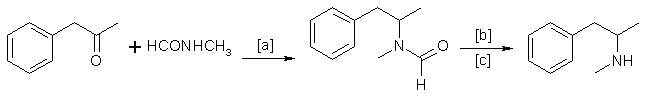
in which [a]: 170-190°C; [b]: H2SO4 or HCl; [c]: 120-170°C. Instead of N-methylformamide, a mixture of methylamine and formic acid is sometimes used23.
Of far lesser importance are the syntheses, in which ephedrine is used as the starting material. Several routes using ephedrine have been reported, including: (a) hydro genating ephedrine12 in acidic solutions using Pd/ BaSO4 and H2 at elevated temperatures (100°C); (b) reacting ephedrine with chlorine (from thionyl chloride or phosphorus pentachloride) and subsequent hydrogenation of the intermediate20 1-phenyl-1-chloro-2- methylaminopropane; (c) reducing of ephedrine with hydrogen iodide and red phosphorus20.
A typical example of thoughtless chemical handling is the reaction in which benzylmagnesium chloride is refluxed with the condensation product of methylamine and acetaldehyde. Due to imperfect chemical handling the efforts to produce methamphetamine were fruitless8.
A wide variety of methamphetamine syntheses are known but of lesser importance. For further details, refer to references3,32.
Survey of Impurities
The impurities reported in the literature are given here according to the sequence developed above on the syntheses of amphetamine and methamphetamine.
| Table 1. Impurities found in amphetamine synthesized with the Leuckart reaction |
||
| Name | Remarks |
Ref |
| Formamide | - |
|
| Formic Acid | ||
| Phenylacetone | ||
| N-formylamphetamine | ||
| 4-Methyl-5-phenylpyrimidine | ||
| 4-Benzylpyrimidine | ||
| Dibenzyl ketone | ||
| alpha-Benzylphenethylamine | ||
| N,N-Di(beta-phenylisopropyl)amine | ||
| 2,4-Dimethyl-3,5-diphenylpyridine | ||
| 2,6-Dimethyl-3,5-diphenylpyridine | ||
| 4-Methyl-5-phenyl-2-(phenylmethyl)-pyridine | ||
| 2-Methyl-3-Phenyl-6-(phenylmethyl)-pyridine | ||
| N,N-Di(beta-phenylisopropyl)methylamine | ||
| 2,4-Dimethyl-3-phenyl-6-(phenylmethyl)-pyridine | ||
| 2-Methyl-2-phenylmethyl-5-phenyl-2,3-dihydropyrid-4-one | ||
| N,N-Di(beta-phenylisopropyl)formamide | ||
| Table 2. Impurities found in amphetamine synthesized by the oxime/phenylnitropropene routes |
||
| Name | Remarks |
Ref |
| 2-Phenyl-methylaziridine | ||
| 2-Methyl-3-phenyl-aziridine | ||
| Benzyl Methyl ketoxime | ||
| Phenyl-2-nitropropene | ||
| Table 3. Impurities found in amphetamine synthesized by the reductive amination of P2P |
||
| Name | Remarks |
Ref |
| Benzyl methyl ketone | ||
| N-Acetylamphetamine | ||
| Dibenzylketone | ||
| N-(beta-Phenylisopropyl)-benzaldimine | ||
| N-(beta-Phenylisopropyl)-benzyl methyl ketimine | ||
| N,N-Di-(beta-Phenylisopropyl)amine | ||
| 1-Oxo-1-Phenyl-2-(beta-Phenylisopropylimino)propane | ||
| 2,4-dihydroxy-1,5-diphenyl-4-methylpentene | ||
| Table 4. Impurities found in methamphetamine synthesized by the reductive amination of P2P |
||
| Name | Remarks |
Ref |
| Benzyl methyl ketone | ||
| Amphetamine | ||
| 1-Phenyl-2-propanol | ||
| N,N-Dimethylamphetamine | ||
| Dibenzylketone | ||
| Table 5. Impurities found in amphetamine synthesized with the Leuckart reaction |
||
| Name | Remarks |
Ref |
| Methylamine | ||
| Formic Acid | ||
| N-Methylformamide | ||
| Benzyl Methyl Ketone | ||
| Amphetamine | ||
| N,N-Dimethylamphetamine | ||
| N-Formylamphetamine | ||
| N-Formylmethamphetamine | ||
| Dibenzylketone | ||
| alpha-Benzyl-N-methylphenethylamine | ||
| N,N-Di-(beta-phenylisopropyl)amine | ||
| N,N-Di-(beta-phenylisopropyl)methylamine | ||
| Table 6. Impurities found in amphetamine synthesized with Ephedrine |
||
| Name | Remarks |
Ref |
| Benzyl Methyl Ketone | ||
| 1,2-Dimethyl-3-phenylaziridine | ||
| 1-Phenyl-2-methylaminopropanone | ||
| Ephedrine | ||
| 1-Chloro-1-phenyl-2-methylaminopropane | ||
- Starting Material
- Intermediate Product
- Product of Side Reaction
- "Route Specific" Impurity
- Impurity in Starting Material
- Found in Phenylnitropropene route only
Conclusions
On the basis of the number and combination of impurities present in the drug preparation and in the case of Leuckart amphetamine c.q. methamphetamine of "reaction specific" impurities, we can trace the synthesis of amphetamine or methamphetamine followed by the illegal producers. Furthermore it is possible to decide (based on the similarity of impurity patterns) that illicit amphetamine preparations originate from the same production batch34. In the cited study on the Leuckart reaction of amphetamine, the effects of the reaction conditions on the production of known impurities were investigated and the possibilities examined for different chemists to produce amphetamine with the same impurity profile by rigidly following the same detailed synthetic directions. Strong support was found in this study for the above stated assumption. Nonetheless the assumption of the equality of batches is tied to the presence of quite a lot of impurities, in which the Leuckart reaction excels, because of its condensation character, where numerous reaction pathways can be followed. With other types of syntheses, impurity signatures can give less information content, as fewer impurities can be present, due to the possibilities for a given reaction. The meaning of equality of profiles under such circumstances should, of course, be approached by another standard of value than in Leuckart type synthesis of amphetamine.
The fact that the identity of the impurities are known will stimulate studies in other unexplored areas, for instance, the acute toxicity of alpha-benzyl-N-methylphenethylamine and alpha-benzylphenethylamine30. The alpha-benzyl components appeared to have a greater CNS stimulation at the brain stem and cord levels than amphetamine. Further studies in this field are expected to appear in due time.
Recent interest has been focused on the nature and level of inorganic trace impurities present in the final product of the methamphetamine synthesis. By using inductively coupled plasma mass spectrometry (ICP-MS) or a combination of ion chromatography and ICP-MS19,39 several inorganic trace impurities in methamphetamine were detected. It appeared even possible to differentiate in this way between two methods of synthesizing methamphetamine. It is expected that further developments in this aspect will take place in due time.