(Master Searcher)
05-19-02 19:17
No 311068
(Rated as: excellent)
One method of reducing amides to amines is by electrochemical reduction. N-substituted amides reduce more readily than the unsubstituted compounds1*. I found this in Ber. Vol. 32 (1899) page 72.
Dimethylbenzylamin aus Dimethylbenzamid.
3 g Dimethylbenzamid in 30 g 50-procentiger Schwefelsäure. Im Anodenraum 20-procentige Schwefelsäure. 2 Ampere. 35º ohne Kühlung. 10 Stunden. Die mit Wasser verdünnte Reactionsflüssigkeit wurde mit Aether extrahirt, welcher 0.6 g unverändertes Amid aufnahm. Die saure Lösung wurde, wie beim Benzylamin beschrieben, weiter behandelt. Die Ausbeute an reinem Dimethylbenzylamin vom Sdp. 180-181º unter 749 mm Druck (Faden ganz im Dampf) betrug 1.3 g, also 63 pCt. der theoretisch berechneten Menge.C9H13N. Ber. N 10.30.. Gef. N 10.34
Das Dimethylbenzylamin ist von Jackson und Wing1) aus Benzylchlorid und Dimethylamin erhalten worden. Sie geben den Siedepunkt zu 183-184º bei 765 mm Druck an und beschreiben die Base als in Wasser unlöslch. In Wirklichkeit löst sich das Dimethylbenzylamin in kaltem Wasser in ziemlich reichlicher Menge und die kalt gesättigte, stark alkalisch reagirende Lösung trübt sich bei gelindem Erwärmen unter Abscheidung der Base. Beim Abkühlen verschwindet die Trübung wieder. Die Löslichkeit der Base in Wasser nimmt also mit steigender Temperatur stark ab. Mit Wasserdämpfen ist der eigenthümlich aromatisch riechende Körper sehr leicht flüchtig.
1.3 g of benzylamine from 3 g of benzylamide. Anybody want to translate this? They recommend using a lead cathode in the article which is known to have a high over voltage. The Berichte article is given as a reference in the two references listed below:
*1. J. Gen. Chem. (USSR) 11, 51 (1941)
2. An Ouline of Organic Nitrogen Compounds E. F. Degering 1950
Also see Post 299552 (PolytheneSam: "Electrochemical reductive amination", Novel Discourse)
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Master Searcher)
05-22-02 17:27
No 312706
All of a sudden I'm getting Cyrillic letters where I had Roman letters with umlauts on them. I found one patent that explains a lot of details of electrochemical reduction of amides. Titanium compounds are added to increase yields of amines and decrease the formation of alcohols. Patent US4695352
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Chief Bee)
05-23-02 08:51
No 313128
> All of a sudden I'm getting Cyrillic letters
> where I had Roman letters with umlauts on them.
Sam: If you are using IE, go to View > Encoding > Western European. You have probably been visiting the Russian HyperLab.
(Moderator)
05-23-02 11:50
No 313248
I've been playing around with the software...
(Master Searcher)
05-26-02 15:46
No 314553
One problem I thought of recently is that indoles tend to be sensitive to strong acids and protonation of the amide seems to be an important step when reducing it electrochemically to an amine. Maybe a weak acid would work in place of the strong acid. You could compare pKa's of various acids. I was thinking of monopotassium phosphate (KH2PO4) or dipotassium phosphate (K2HPO4). K3PO4 works good as an electrolyte for electrochemical reduction of nitriles.
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Master Searcher)
05-28-02 16:22
No 315347
I thought of something else. Acid is used as a catalyst in the Fisher synthesis (phenylhydrazones to indoles) so indoles must be somewhat stable in the presence of acids (also Lewis acids such as ZnCl2), ie. dilute acids. Here's some examples.
../rhodium /dmten.h
Patent US1866956
Patent US2057948
Patent US2068800
Patent US3557142
Patent US4965369
Patent US5229413
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious
(Newbee)
05-30-02 15:19
No 316071
What about acetamide being reduced to ethylamine? If it could be dreampt of industrially, then...
(Master Searcher)
06-02-02 15:14
No 316920
Here's something from two references I found.
The reduction is carried out in closed porous cells, with lead electrodes, and with 20% sulfuric acid as the anode liquid. The cathode solution is prepared by dissolving the amide in a mixture of alcohol, water and sulfuric acid. Formamide, acetamide and N,N-dimethylpropionamide are very resistant to reduction, but the N-substituted amides reduce more readily than do the parent compounds.88
88 J. Gen. Chem. 11, 51, (1941)
from An Outline of Organic Nitrogen Compounds by E. F. Degering 1950
Reduction Reduction of amides has been mentioned in Chapter 2 as a useful preparative method for amines. Mild reducing agents do not affect amides. Electrolytic reduction,24 usually with lead or zinc amalgam electrodes in sulfuric acid solution, used to be the best method, although it is generally successful only with amides of aromatic acids and with dialkylamides of aliphatic acids. Hydrogenation can also be useful, but the general inertness of amides makes high pressures and temperatures necessary. Recent studies 25 have shown rhenium to be an unusually effective catalyst, however. Sodium and hot alcohol, a reducing system of wide application, is of very limited value with amides. Many amides are little affected, and those that do react undergo C-N cleavage to a major extent, producing the alcohol derived from the acyl group. So-called Birch reduction-sodium, calcium, etc., in liquid ammonia plus a proton source, such as alcohols-produces aldehydes, 26 and serves as a useful deacylating method.
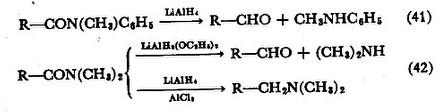
The metal hydrides, essentially lithium aluminum hydride and its derivatives, are the foremost reducing agents for amides. With proper choice of reagent and proportions, either amines or aldehydes can be obtained in preparatively useful yields (Eq. 40). Reduction apparently
goes in two stages, first by addition of hydride to the carbonyl group, forming a carbinolamine (as its aluminate complex), which can be hydrolyzed to an aldehyde. In the next stage, either a C-N or a C-O bond must be broken, resulting respectively in an alcohol, or an amine. Of course, it is only when the first stage is faster than the second that it is possible to stop the reduction at the carbinolamine stage for the purpose of aldehyde synthesis. Such conditions prevail with N-methylanilides in general when lithium aluminum hydride is used,27 and with dialkylamides when lithium diethoxyaluminum hydride, LiAlH2(OC2H5)2, is the reducing agent 28 (Eqs. 41, 42). Anhydrous aluminum chloride
used with lithium aluminum hydride greatly favors C-O cleavage in the second reduction stage, and thus improves the conversion of amides to amines29 (Eq. 42). Unsubstituted amides appear to be dehydrated in the first stage of reaction with lithium aluminum hydride, giving nitriles, which are, of course, reduced rapidly to amines.30 With such a reaction path, aldehyde formation of the type described above cannot occur, and yields of amines are accordingly high.
from The Chemistry of Open Chain Organic Nitrogen Compounds, Vol. 1, P. A. S. Smith, 1965
http://www.geocities.com/dritte123/PSPF.
The hardest thing to explain is the obvious