(Chief Bee)
02-13-04 11:54
No 488336
(Rated as: excellent)
Die Synthese von Naturstoffen, insbesondere von Alkaloiden, unter physiologischen Bedingungen und ihre Bedeutung für die Frage der Entstehung einiger pflanzlicher Naturstoffe in der Zelle
IV. Die Synthese der Tropaalkaloide and des Pseudopelletierins unter physiologischen Bedingungen.
Prof. Dr. Clemens Schöpf
Angewandte Chemie, Vol 50, No. 40, pp. 779-790 (1937) (../rhodium/pdf /tropinone.an
Summary
A section about the Robinson synthesis of tropinone from a review article on alkaloids, prepared under physiological reaction conditions (no exotic catalysts, near neutral pH, etc.). It shows very nice graphs of the relationship between the solution pH and the yield of tropinone isolated from a mixture of acetonedicarboxylic acid, succindialdehyde and methylamine allowed to stand at room temp for 72h. The graph peaks at 80% at pH=5, but the yield stays at 60%+ all the way between pH 3-11.
____ ___ __ _
One-pot synthesis of tropinone by tandem (domino) ene-type reactions of acetone silyl enol ethers
Koichi Mikami and Hirofumi Ohmura
Chem. Commun. (22), 2626-2627 (2002) (../rhodium/pdf /tropinone-ac
DOI:10.1039/b208066d
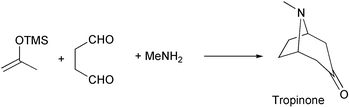
Abstract
A synthetic approach for tropane alkaloids on the basis of tandem (domino) ene-type reactions of acetone silyl enol ethers with iminium ions is shown to be triggered by intermolecular ene-type reactions followed by 6-(2,5)silatropic ene-type cyclizations.
____ ___ __ _
Concerning 2-Carbomethoxytropinone
Steven P. Findlay
J. Org. Chem. 22, 1389-1394 (1957) (../rhodium/pdf /2-carbometho
Abstract
Racemic 2-carbomethoxytropinone is obtainable by the partial saponification of 2,4-dicarbomethoxytropinone and, more conveniently, by the condensation of monomethyl beta-ketoglutarate, got from beta-ketoglutaric anhydride, with succindialdehyde and methylamine. 2-Carbomethoxytropinone can conceivably exist in three racemic and six optically active forms. Of these only one has been obtained heretofore. The configurational relation of d-(2-carbomethoxytropinone) and its l antipode to l-cocaine is established by the Kiliani chromic acid oxidation of pseudoecgonine methyl ester to the former. Previous methods of preparing racemic 2-carbomethoxytropinone, the properties of 2,4-dicarbomethoxytropinone, and incidental experimental data are discussed.
____ ___ __ _
Stereoselective reduction of Tropinone and 2-Carbomethoxytropinone
Post 458047 (Rhodium: "Stereochemistry of the Reduction of Tropinone", Serious Chemistry)
Post 458140 (roger2003: "2-Carbomethoxytropinone to Methyl Ecgonine", Serious Chemistry)
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
03-14-04 22:26
No 495136
(Rated as: excellent)
The Effects of Microwave Irradiation on Occluded Solvents in Illicitly Produced Cocaine Hydrochloride
David R. Morello, John F. Casale, Margaret L. Stevenson, and Robert F. X. Klein
J Forensic Sci, Vol. 45, No. 5, pp. 1126-1132 (2000) (../rhodium/pdf /cocaine.micr
Abstract
The current clandestine methodology for the manufacture of illicit cocaine hydrochloride utilizes microwave heating in order to dry the finished product. This study addresses the effects this step has on the occluded solvents present in newly prepared cocaine hydrochloride. Nine 1-kilogram-sized batches of cocaine hydrochloride were prepared from cocaine base using a variety of solvents or solvent mixtures commonly utilized in clandestine laboratories, pressed into bricks, and submitted to microwave heating. Residual solvents were qualitatively and quantitatively monitored before, during, and following the microwaving step by static headspace-gas chromatography-mass spectrometry. All solvents used in the conversion process were easily detected in the bricks even after extensive irradiation, confirming that occluded solvents are extremely resistant to removal by microwave heating. Qualitative and quantitative data corresponding to the residual solvents in the prepared cocaine hydrochloride bricks are presented.
____ ___ __ _
Comparative Determination of 2-Carbomethoxy-3-Alkyloxy- and Heteroaroyloxy-Substituted Tropanes in Illicit South American Cocaine Using Capillary Gas Chromatography-Single Ion Monitoring
John F. Casale, James M. Moore, and Norman G. Odeneal
J Forensic Sci, Vol. 43, No. 1, pp. 125-132 (1998) (../rhodium/pdf /cocaine.trac
____ ___ __ _
Detection of Cocaine on Various Denominations of United States Currency
Adam Negrusz, Jennifer L. Perry, Christine M. Moore
J Forensic Sci, Vol. 43, No. 3, pp. 626-629 (1998) (../rhodium/pdf /cocaine.curr
____ ___ __ _
Identification of cis- and trans-Cinnamoylcocaine in Illicit Cocaine Seizures
James M. Moore
J. Ass. Off. Anal. Chem. 56(5), 1199-1205 (1973) (../rhodium/pdf /cinnamoylcoc
Abstract
During the in-depth analysis of illicit cocaine samples small amounts of other coca alkaloids and cocaine degradation products have been detected. One of these alkaloids, cinnamoylcocaine, has been found in more than half of the samples examined, usually in concentrations of 1% or less of the amount of cocaine present. The presence of cinnamoylcocaine, as its cis and trans isomers, was established by column partition chromatographic isolation of the isomers, followed by ultraviolet, infrared, nuclear magnetic resonance, and mass spectrometric identification.
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
03-17-04 01:20
No 495587
(Rated as: good read)
Ueberführung von Tropinon in r-Cocaïn
R. Willstätter & A. Bode
Chem. Ber. 34, 1457-1461 (1901) (../rhodium/pdf /cocaine.will
____ ___ __ _
Bildung von Tropin aus Tropidin und die Synthese des Atropins
A. Ladenburg
Chem. Ber. 35, 1159-1162 (1902) (../rhodium/pdf /tropine-trop
____ ___ __ _
Ueber Pseudotropin
Richard Willstätter
Chem. Ber. 29, 936-947 (1896) (../rhodium/pdf /pseudotropin
____ ___ __ _
Ein Neuer Synthetischer weg in der Tropan-Reihe
C. Grundmann & G. Ottmann
Ann. Chem. 605, 24-32 (1957) (../rhodium/pdf /anhydroecgon
Summary:
Racemic anhydroecgonine is prepared by addition of methylamine to cycloheptatriene-carboxylic acid, which itself is prepared by the addition of ethyl diazoacetate to benzene [Ann. Chem. 582, 163 (1953)].
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
03-19-04 16:14
No 496140
(Rated as: good read)
A Synthesis of Tropinone
Robert Robinson
J. Chem. Soc. 762-768 (1917) (../rhodium/pdf /tropinone.ro
____ ___ __ _
Ueber das Tropinon
Richard Willstätter
Chem. Ber. 393-403 (1896) (../rhodium/pdf /tropinone.wi
____ ___ __ _
Ueber das dritte racemische Cocain
Karl Zeile & Werner Schulz
Chem. Ber. 678-679 (1956) (../rhodium/pdf /dritte.racem
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
03-19-04 17:28
No 496152
(Rated as: good read)
2-carbomethoxytropinone from succindialdehyde, methylamin and Ethyl acetoacetate
Patent DE345759
(Hive Bee)
09-05-04 18:51
No 529695
(Rated as: excellent)
Total Synthesis of (+)-Cocaine via Desymmetrization of a meso-Dialdehyde
Douglas M. Mans and William H. Pearson
Org. Lett.; 2004; ASAP Web Release Date: 19-Aug-2004
DOI:10.1021/ol048777a

Experimental procedures: http://pubs.acs.org/subscribe/journals/o
NMR spectra: http://pubs.acs.org/subscribe/journals/o
Abstract: The total synthesis of (+)-cocaine is described. An extension of the recently reported proline catalyzed intramolecular enol-exo-aldol reaction to a meso-dialdehyde provided the tropane ring skeleton directly with good enantiomeric excess. The meso-dialdehyde was prepared using a 2-azaallyllithium [3 + 2] cycloaddition to generate a cis-2,5-disubstituted pyrrolidine. Overall, the synthesis proceeded in 6.5% yield and 86% ee over 14 linear steps starting from commercially available 3-benzyloxy-1-propanol.
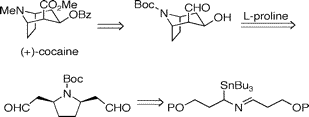
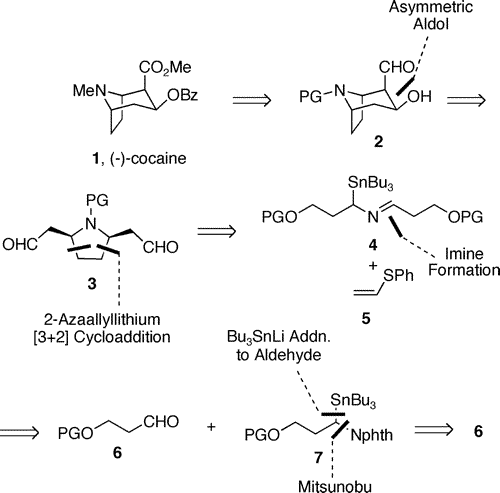
Scheme 1. Retrosynthesis of Cocaine
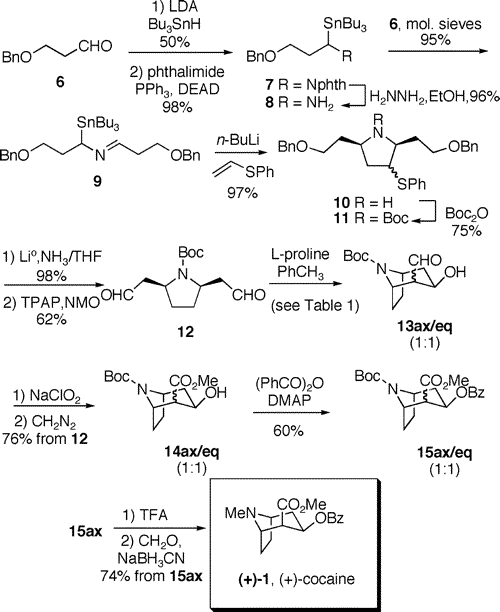
Scheme 2. Total Synthesis of (+)-Cocaine
The tendency is to push it as far as you can
(Chief Bee)
10-06-04 05:09
No 534620
(Rated as: good read)
Cocain-Synthese aus Hyoscyamin. I. Darstellung von Tropinon-carbonsäure-estern
N. A. Preobrashenski, M. N. Schtschukina, R. A. Lapina
Chem. Ber. 69, 1615-1618 (1936) (../rhodium/pdf /hyoscyamine2
____ ___ __ _
Über ein Nebenalkaloid des Cocains, das Isatropylcocain
C. Liebermann
Chem. Ber. 21, 2342-2355 (1888) (../rhodium/pdf /truxilline.l
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
10-06-04 06:32
No 534634
I am very interested in these two "Cocaine Precursors from Hyoscyamine" papers. Could someone translate the experimental sections of them?
Crank is part of this complete breakfast.
(Chief Bee)
10-06-04 17:53
No 534685
Preobrashenski only describes the hydrolysis of Hyoscyamine to tropine and its oxidation to tropinone in very general terms with references to other articles, and the actual experimental part only details the carboxymethylation of tropinone with sodium and dimethylcarbonate (described more in detail by S. P. Findlay in his landmark article Concerning 2-Carbomethoxytropinone, see Post 488336 (Rhodium: "Synthesis of Tropinone & 2-CMT", Methods Discourse)).
The second article in my post above discusses α/β-Truxillines and other cocaine-related alkaloids from Erythroxylon coca.
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
10-07-04 04:18
No 534772
OK... thanks for the info. Good read. Is it possible to produce 2,5-Diethoxytetrahydrofuran from 2,5-Dimethoxytetrahydrofuran? I probed the search engine and didn't find anything on this.
Crank is part of this complete breakfast.
(Chief Bee)
10-07-04 05:21
No 534778
What use could you have for the diethoxy which cannot be done with the dimethoxy?
The Hive - Clandestine Chemists Without Borders
(Hive Bee)
10-07-04 05:40
No 534780
Since they are so similar it seemed like they where interchangeable, but I wasn't 100% sure. I'm glad I can use it though... as its OTC. SWIDP is getting really close to an OTC Cocaine synth with the exception of a few chems. If SWIDP could just get a few pure grams it would make them so damn happy!
Crank is part of this complete breakfast.
(Chief Bee)
10-15-04 00:53
No 535874
(Rated as: excellent)
The Cocaine Diastereoisomers
A. C. Allen, D. A. Cooper, W. O. Kiser, R. C. Cottrell
J. Forensic Sci. 26(1), 12-26 (1981) (../rhodium /cocain
Abstract
In the past, it has been argued in court, from a theoretical basis, that the techniques available to the forensic chemist would differentiate the "cocaines". This work has moved that argument from the realm of the theoretical into that of experimental fact. The techniques of infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and mass spectrometry (MS) will unequivocally identify the racemic cocaine diastereoisomer. In addition, this work shows that the enantiomeric form of cocaine can be assigned by crystal tests, IR, and melting point techniques. The pure enantiomers of allococaine and pseudoallococaine were not isolated. This does not create a problem because the techniques of NMR and MS, as performed in this study, will not differentiate enantiomers. Therefore, the logical sequence of first identifying the diastereoisomer (via IR, NMR, or MS) and then determining the chirality by crystal tests, IR, melting points, or optical rotation measurements is valid.
____ ___ __ _
The Three-dimensional Structure of the Cocaines. Part I.
Cocaine and Pseudococaine
Stephen P. Findlay
J. Am. Chem. Soc. 76, 2855-2862 (1953) (../rhodium/pdf /cocaine.pseu
Abstract
Published experimental data on the chemistry of cocaine and its simpler derivatives are interpreted as indicating that this base is 2β-carbomethoxy-3β-benzoxytrop
____ ___ __ _
The Three-Dimensional Structures of the Cocaines. Part II.
Racemic Allococaine and Racemic Allopseudococaine
Stephen P. Findlay
J. Org. Chem. 24, 1540-1550 (1959) (../rhodium/pdf /allococaine.
Abstract
Catalytic hydrogenation of racemic 2-carbomethoxytropinone in acetic acid yields racemic alloecgonine methyl ester, which can be transformed to the racemates of alloecgonine, allococaine, allopseudoecgonine, allopseudoecgonine methyl ester, and allopseudococaine. Some limitations of a generalization concerning the course of the catalytic hydrogenation of cyclic ketones as it applies to certain keto derivatives of the tropane and morphine alkaloids are noted. The three-dimensional structures of the new cocaines are tentatively assigned. The possible utility of molecular rotation data in ascertaining the absolute configuration of transformation products of the 2-carbomethoxy derivatives of both tropinone and N-methyl-granatonine is indicated. Some other possible methods of synthesizing the new cocaine isomers and the drawbacks thereof are mentioned.
____ ___ __ _
The Synthesis of Racemic Allococaine and Racemic Allopseudococaine
Stephen P. Findlay
J. Org. Chem. 21, 711 (1956) (../rhodium/pdf /allococaine.
____ ___ __ _
The Conversion of Certain Pyrroles to α,δ-Alkanedioximes
Stephen P. Findlay
J. Org. Chem. 21, 644-647 (1956) (../rhodium/pdf /pyrrole2succ
Abstract
Contrary to the general report, hydroxylamine alone does not convert pyrrole to succindialdoxime. Hydroxylamine hydrochloride alone is likewise ineffective. However, equivalent amounts of these substances (Lossen’s hydroxylamine hemichloride) do effect the conversion. The action of these substances on 2,5-dimethylpyrrole is similar. An improved procedure for the preparation of succindialdoxime and certain of its properties are described.
For Tropinone Chemistry, see:
Post 482456 (Lego: "Mechanism of Robinson’s synthesis of tropinone", Serious Chemistry)
Post 433727 (Megatherium: "Robinson-Schöpf reaction: tropinone", Chemistry Discourse)
Post 338869 (Tricky: "Robinson's tropinone: improving method!", Novel Discourse)
The Hive - Clandestine Chemists Without Borders
(Chief Bee)
10-16-04 04:31
No 536032
(Rated as: excellent)
Synthesis of Cocaine
G. I. Bazilevskaya, M. S. Bainova, D. V. Gura, K. M. Dyumaev, and N. A. Preobrazhenskii
CA 53, 423h (1959) [Izvest. Vysshikh Ucheb. Zavedenii, Khim. i Khim. Tekhnol. 75-81 (1958)]
To a mixt. of 32.2 g. furan, 95 mL dry Et2O, and 145 mL anhyd. EtOH, cooled to -35°C, is added, dropwise, during 1 h with stirring a soln. of 24.2 mL Br2 in 335 mL EtOH also cooled to -35°C, while keeping the soln. below -25°C. After 30 min. standing, dry NH3 is added up to pH 6, the mass stirred at -5°C until the color disappears and again NH3 added up to pH 8 to give 2,5-diethoxy-2,5-dihydrofuran (I) (52.6 g), bp3 39-41°C, d20 1.0017, nD20 1.4310. The dimethoxy analog, bp17 69.74°C, d20 1.0730, nD20 1.4352, may be obtained in a similar manner except that the reaction is conducted in the absence of Et2O (yield: 71%).
I (47.5 g) is hydrogenated in the presence of 5 g of Raney Ni at room temp. and at atm. pressure with stirring. After the absorption of 7.2 L H2 during 2-3 h, the catalyst is filtered off and washed with 15 mL dry EtOH; 40.1 g 2,5-diethoxytetrahydrofuran (II), bp20 76-78°C, d20 0.9630, nD20 1.4193, is thus obtained. The methoxy analog, bp22 52-54°C, d20 1.0230, nD20 1.4178, is similarly obtained (yield: 85.5%).
To a mixt. of 360 g 50% KOH soln. and 138 mL MeOH, 70.5 g dimethyl ester of acetonedicarboxylic acid (III) is added with stirring at -5°C. The temp. rises immediately to 15°C, and then up to 25°C during 30 min. After 10 min. standing the mixt. is again cooled to 0°C and 65 mL Et2O is added. The ppt. is filtered off and washed with 65 mL MeOH and 150 mL Et2O (previously cooled to 0°C). The di-potassium salt (86.2 g) of III is obtained.
To 1 N HCl (322 mL) heated to 80°C is added 41.1 g II and the mixt. stirred during 20 min., rapidly cooled to 10°C, and 211 mL 1N HCl, 98.2 g III, 26.4 g AcONa, and 28.2 g CH3NH2·HCl added. The mixt. is stirred 4 h at 29-31°C, cooled to 10°C, satd. with 410 g KOH, and extracted 4 times with CHCl3 (75 mL, 15 min. stirring). The methyl ester (IV) (25.96 g), mp 106-107°C (from MeOH), bp0.2 85-86°C, of tropan-3-one-2-carboxylic acid crystallizes from the oily mixt. (2.88 g more is obtained from the mother soln.); IV·HCl, mp 172-173°C (from MeOH); IV·H2O, mp 97-100°C.
IV (28.34 g.) is dissolved in 10% H2SO4 (170 mL), cooled to -5°C, and treated with 3.63 kg 1.5% Na-Hg with vigorous stirring between -2°C and +2°C, the pH being kept at 3-3.5 by means of a 30% H2SO4 soln. The reduction is continued about ½ hr. until 3 drops of the reacting mixt. cease to give a red coloration with a 10% soln. of FeCl3. After the sepn. of Hg, the soln. is satd. with 235 g KOH below 15°C and extd. with CHCl3 (250 ml., 5 times). The extracts are dried over Na2SO4 and an oily liquid (26.5 g) is obtained, from which the Me ester of racemic pseudoecgonine (V) crystallizes upon long standing (5-7 days at 0°C).
The 2 isomeric esters of ecgonine, V and racemic ecgonine (VI), are sepd. by mixing the oily liquid (filled with crystals) with an equal volume of dry Et2O. The pptd. V (5.86 g), mp 128.5-130.5°C (from Et acetate), is filtered off. Its HCl salt mp 211-213°C. To the filtrate is added 250 ml. dry Et2O until no more ppt. forms (the ppt. rapidly melts in the air to form a resinous mass), and the filtrate is stirred 30 min. with activated coal. The solvents are evapd. and a light brown liquid (17.2 g) is obtained; it is dissolved in 17 ml. MeOH and neutralized with a 10% soln. of HCl in dry Et2O. Et2O is then evapd. in vacuo until the 2 layers disappear. Upon standing 2 hrs. at 0°C, VI·HCl crystallizes; it is filtered and washed with a mixt. 1:1 MeOH-dry Et2O cooled to 0°C. Pure VI·HCl, mp 194.5°C, is obtained upon recrystg. from MeOH and washing with small quantities of 1:1 MeOH-Et2O and then with Et2O; 1.55 g. more VI·HCl may be obtained from the mother soln. (total yield 9.85 g).
IV·HCl (9.33 g) is heated 10 h on a water bath with 18.7 g PhCOCl, the brown transparent liquid formed is poured into 250 mL Et2O, and upon rubbing, the viscous mass is converted into a friable powder which is dissolved in 35 ml. ice water and neutralized to the universal indicator by 20% NH4OH. Racemic cocaine (VII) (base) (6.81 g) mp 80-81°C (from ether), is filtered off, washed with 12 mL of ice water and dried over CaCl2. A still larger yield of VII (84% calcd. from VI) is obtained by treating the mother soln. VII·HCl, m. 186-187°C, is obtained by exactly neutralizing the soln. of the base in a sevenfold quantity of Et2O with an alc. soln. of HCl, followed by washing the crystals with 1:3 MeOH-Et2O and then with Et2O.
The Hive - Clandestine Chemists Without Borders